Understanding Oxyhemoglobin
by Peter Bonadonna
The Oxygen molecule, labeled O, when unbound, is referred to as a singlet of oxygen. This form of oxygen in excess in the body is undesirable and can have damaging effects. Small amounts are normally produced as a result of normal metabolism. In the body, singlet oxygen is often called a free radical. Above is an illustration of oxygen in its singlet form (left) and as a gas(right). Two oxygen molecules are bound together to form O2, a gas essential for human and animal life. If three O molecules are bound together you would have O3, also called ozone.
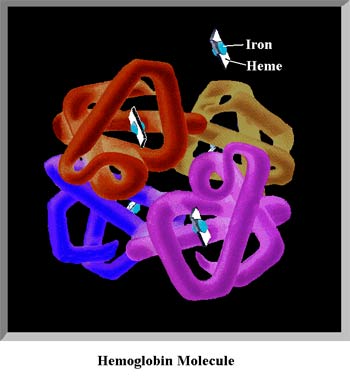
This is one of natures most complicated and unique creations, a protein molecule called hemoglobin. Each hemoglobin molecule is made up of 10,000 atoms, four of which are Iron atoms (blue spheres in the figure) that act as magnets to attract and hold the oxygen molecules. Each Iron atom rests on a Heme platform which serves to release the oxygen, out in the peripheral tissues. Each Red Blood Cell contains about 250 million molecules of hemoglobin, each cc of blood contains 5 billion Red Blood cells, you have approximately 5,000 cc's of blood in your vascular system. The reason we have so much hemoglobin is because oxygen does not easily dissolve in water (about 3% of all our oxygen is in the serum - the rest is bound to hemoglobin), so we have developed this unique system of oxygen transportation to meet our needs.
When oxygen is bound to hemoglobin, it is called oxyhemoglobin.
The air around us is made up of many substances. The major gasses are: nitrogen, oxygen, carbon dioxide, water vapor. The minor gasses (accounting for less than 1%) are argon, xenon, ozone, carbon monoxide, nitrous oxide, helium and so on. Other substances float around in this "sea" of gasses such as organic and inorganic dusts and vapors.
The principle or most abundant gas is nitrogen (N2) accounting for 78 % of the molecules in an air sample. Nitrogen is a colorless, odorless, inert gas. This means that it does not easily react with other chemicals. This does not mean that nitrogen is unnecessary to us. We do absorb nitrogen from the air, and it is necessary for normal life functions. At the alveolar membrane, nitrogen molecules are constantly moving into and out of our blood. There is an equilibrium reached between our body and the nitrogen in the atmosphere. You can, however, go short periods without breathing nitrogen. When you give your patient 100% oxygen, the patient is deprived of the nitrogen in room air. Gradually, your body exhales the nitrogen present in the cells and blood stream. Remember your lessons on diffusion? If the alveolus is filled with 100% oxygen, then the nitrogen in the blood moves from the area of higher concentration to an area of lower concentration. Because the airway is being flushed with pure oxygen, there is a large gradient between the capillary bed and alveolus. A large amount of nitrogen can be removed in a short time.
If a patient were to receive pure oxygen for days, nearly all of the body's nitrogen gas would be removed. You have probably heard of Oxygen Toxicity, it is a misnomer because most of the damage occurs from a deficiency of nitrogen. Knowing these principles helps you understand other issues and treatments in medicine. Example: Patients with small, stable pneumothoraces (10-20% collapse) will be sent home by the ED. The pocket of air will gradually be absorbed in a weeks time. Some physicians have the patient Breath 100% oxygen for 30 minutes twice a day for two days. The air trapped in the pleural space is usually absorbed in only 24 hours.
Oxygen or O2 is a colorless, odorless gas that is present in our atmosphere along with other gasses. Oxygen accounts for 20.9 % (often rounded to 21) of the air around us. Oxygen is an essential component in the Citric Acid Cycle. Oxygen is used by the cells in our body to produce the energy needed for heat production and cellular energy. Too much or too little oxygen can cause illness and death. This is why it has become necessary for health care professionals to be able to quantify the amount of oxygen in the blood stream.
Years ago, the only method of determining the oxygen content of arterial blood was to puncture an artery with a needle and send that blood to a lab. The result came back as the "Partial Pressure" of oxygen dissolved in the total sample. This may be a new concept for some and we need to simplify the concept of measuring gasses that are dissolved in a liquid.

At sea level, the atmosphere pushes down on us like the water would if you were on the bottom of the ocean. Even though you don't feel it, your body is being pressed by 14.696 lbs. per square inch or 760 mm Hg. If you were in an atmosphere of pure Oxygen, your arterial Pressure of Oxygen would nearly equal 760 mm of Hg in the blood stream. Because our atmosphere is only 20.9% oxygen, we need to find 20.9% of 760 to determine how much oxygen (or the Partial Pressure)is being forced into our blood. 20.9% of 760 is 158.8 after adjusting for airway dead space, elevation, patient temperature, and water vapor, a healthy patient breathing room air can have a PaO2 that typically ranges from 90-106 Torr. The PaO2 can go much higher than this if the patient is breathing supplemental oxygen and or under increased barometric pressure such as a hyperbaric chamber.
You have probably heard a physician say that a patient's Arterial Blood Gas (ABG) has a PaO2 of 104 mm Hg. (see Figure 1.) In your paramedic text books you have read that an Oxygen of less than 80 mm Hg is considered hypoxia. So you know that this patient is not hypoxic.
There is a relationship between the amount of oxygen dissolved in the blood and the amount attached to the hemoglobin. Below is a diagram which shows this relationship.
Normal Oxyhemoglobin Dissociation Curve
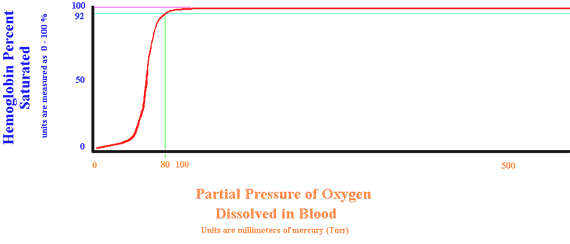
The illustration above shows us that when the partial pressure of oxygen is 80 Torr., the hemoglobin is 92% saturated with oxygen. As the tension (pressure) of oxygen increases, the hemoglobin becomes more saturated. Note that any tension above 105 completely saturates the hemoglobin. You can still drive more oxygen into the blood liquid, but the hemoglobin is completely loaded. Using a pulse oximeter allows us to assess the oxygen tension by looking at hemoglobin saturation.
Unfortunately, it's not that easy. There are several factors that influence hemoglobin attraction to oxygen. The curve can be shifted to the right or left.
Shift to the Left
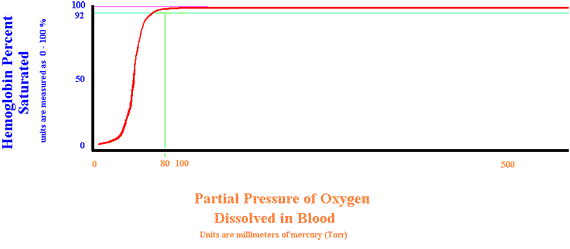
Several situations can increase hemoglobin's affinity for oxygen. (Oxygen strongly binds to hemoglobin and is less available to the tissues) They are: Decreased Hydrogen ion, Decreased PaCO2, Decreased temperature, and Decreased 2-3-DPG. Note that if your pulse oximeter shows a saturation of 95% (Usually considered normal in the range) the patient is now very hypoxic with a PaO2 of 76 Torr.
Shift to the Right
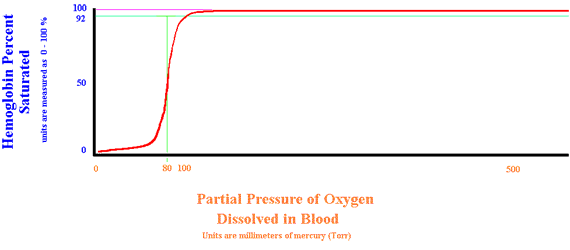
Several situations can decrease hemoglobin's affinity for oxygen. They are: Increased Hydrogen ion, Increased PaCO2, Increased temperature, and Increased 2-3-DPG. This allows hemoglobin to easily off-load oxygen to the peripheral tissues. Note that when a shift to the right occurs, a saturation of 75% (usually considered severe hypoxia) is a PaO2 of 88 Torr. a normal partial pressure.
Two other conditions can cause you to incorrectly assess a patients oxygen status. They are Carbon Monoxide poisoning and anemia. In both cases the pulse oximeter will falsely report a high saturation number when the patient is desperately hypoxic.
Pulse Oximetry is best used by Critical Care Technicians and Paramedics who understand how this technology works, and understand the pitfalls. It is most useful to trend a patients condition. Never reduce, withhold or deny a patient oxygen therapy based on pulse oximetry readings. Use your training and judgement to make the decision. When in doubt.....give oxygen in adequate amounts. Treat the patient....not the number!

